
Thermal Validation
- Home
- Thermal Validation
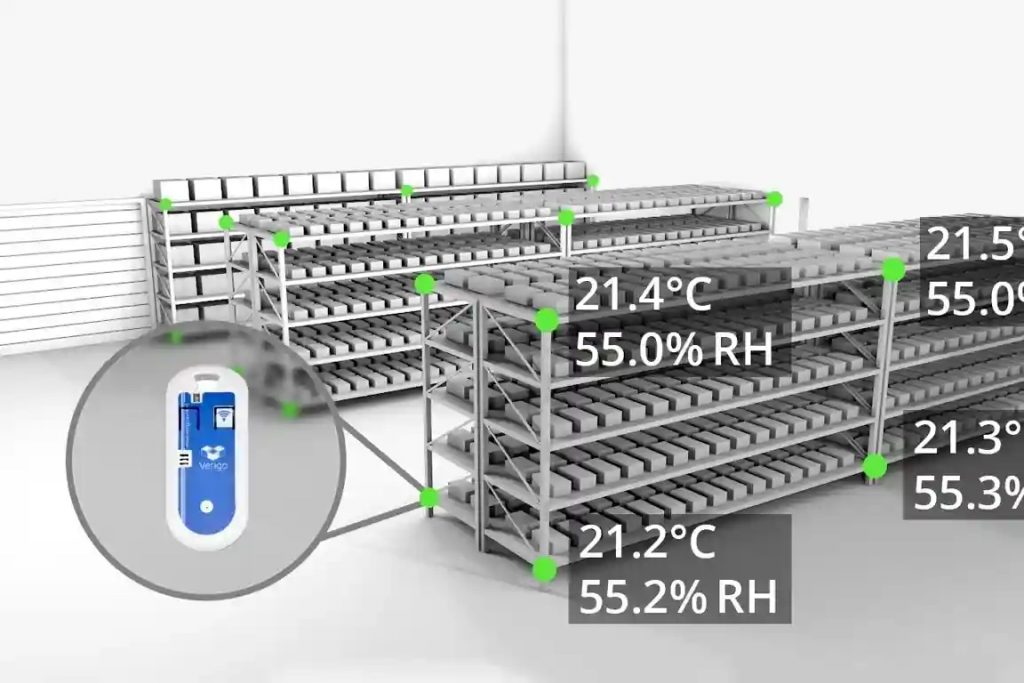
Temperature and Humidity Mapping
A temperature mapping study is an exercise to qualify temperature and humidity mapping of storage environments used for temperature sensitive
products.
This can include but is not limited to Warehouses (Controlled, uncontrolled, Dedicated or Multi Market), Cold stores/Fridges, Freezers, Containers, Vehicles, Stability Chambers, Incubators)
The temperature mapping report will document any
recorded Hot & Cold spots as well as recommendations for continuous monitoring positions where required
Thermal Validation for ETO Sterilizers
EtO sterilization is used on heat sensitive products. Consequently validation exercises must verify that temperature remains within specified parameters. Other critical parameters are pressure and humidity.
Cycle Physical performance Analysis
Temperature and Relative Humidity monitoring
Pressure monitoring

Depyrogenation Validation:
The removal of pyrogens from glass and lab equipment in dry heat ovens or Tunnels is called Depyrogenation.
Most pyrogens are endotoxins, and although endotoxins are relatively thermally stable, a significant reduction in endotoxin levels can be achieved within a dry heat sterilizer.
Heat Distribution and Penetration Study
Cold and Hot Points Detecting
Depyrogenation Period Calculation
( Min , Max , Sterilization Time)
Lethality Calculations

Thermal Validation for Autoclaves
sterilizer thermal validation of autoclaves consists of accurately measuring the temperature at critical points within the autoclave chamber throughout the process
• Heat Distribution and Penetration Study
• Cold and Hot Points Detecting
• Sterilization Period Calculation
• ( Min , Max , Sterilization Time)
• Lethality Calculations
Thermal Validation - Temperature Mapping System

TEMPERATURE MAPPING FOR COLD STORES, VEHICLES, CONTAINERS, CABINETS (FRIDGES, FREEZERS, STABILITY CHAMBERS):
Empty & With load (product)
External ambient temperature (outside of
cabinet/cold store/vehicle)
Date stamp of any notable
events (e.g. power failure)
Impact tests (e.g. door open , Power cut)
Mapped during normal operations

TEMPERATURE MAPPING FOR WAREHOUSES
Empty & With load (product)
Summer & Winter
External ambient temperature (outside of facility)
Date stamp of any notable
events (e.g. power failure)
temperature and humidity mapping during normal operations
Impact tests (e.g. HVAC power down, Door open tests)
MKT (Mean Kinetic Temperature)
Thermal Validation - Temperature Mapping System
Our thermal validation software provides compliance with FDA regulation in 21CFR Part 11 It is providing a Full Automated Reporting and Report Management In addition, software provides all the essential elements for meaningful and successful validation. , all parameters including calibration data associated with a validation study are stored in a specific secured, configuration file, printable for hardcopy filing.
During the test, data is time stamped and stored at a default scan value in an unmodifiable. Operators can view in real time any or all parameters being measured with both graphical and digital displays. The configured validation test data can be printed for hardcopy in both graphical and tabular format. We Let you Know all you need from our Study Report With Clear Thermal Validation Reported contain
Calibration Report and Calibration Check Report – Cycle Analysis – Cycle Mapping Supported With Chart , Chart Zoom , Sensor positioning , Data list – Data list Calculations , and finally you will find Thermal Validation Summary Report